Abstract
JAK2 V617F is the most common driver mutation for Ph-negative MPN and related blast transformation (MPN-BP). In contrast, JAK2 V617F is rare (<5%) in acute myeloid leukemia (AML) lacking an antecedent MPN (nonMPN AML). JAK2 V617F skews the differentiation of hematopoietic stem/progenitor cells (HSPC) towards erythroid and megakaryocytic lineages and in conjunction with TP53 deficiency/mutations results in acute erythroid leukemia in mice (Rampal et al., PNAS 2014). These phenotypes, although also reported in patients with MPN-BP (Mesa et al., Blood 2005), remain incompletely understood. In this study, we set out to study these phenotypes at the protein, epigenomic and transcriptomic levels in JAK2-mutated AML comparing MPN-BP to nonMPN AML.
The study cohort included 58 MPN-BP (16 PV, 16 ET, 14 PMF and 12 MPN-U) and 27 nonMPN AML patients (11 de novo AML, 12 AML-MRC, 4 t-AML) with JAK2 V617F mutation (Table 1). In addition, 100 patients with JAK2 WT AML were also included as control group. The median age at diagnosis in MPN-BP and nonMPN AML subgroups as well as in JAK2 WT AML was similar (70 years vs 71 years vs 70 years, respectively) and showed a male preponderance. The median interval between MPN diagnosis and blast transformation was 4.3 years (range, 0-20.5 years). Overall survival was inferior in MPN-BP compared to nonMPN AML (median survival: 7.4 vs 19.3 months). MPN-BP showed a higher rate of MF-2 or 3 myelofibrosis (78% vs 33%, p<0.05), osteosclerosis (50% vs 15%, p<0.05) and marrow necrosis (7% vs 0%) on bone marrow biopsies than nonMPN AML. On immunohistochemistry, 28% (10/32) MPN-BP cases (vs 0% nonMPN AML, p<0.05) were classified as acute leukemia with erythroid/megakaryocytic differentiation (M6b and/or M7) based on expression of E-cadherin, CD71, Glycophorin A, and CD61 on leukemic blasts. ERG, FLI1, and LMO2, transcription factors that are often expressed in HSPCs, were also expressed in leukemic blasts of MPN-BP and nonMPN AML. Aberrant TP53 expression by immunohistochemistry was seen in 31% of MPN-BP (5/9 and 5/21 cases with and without erythroid/megakaryocytic differentiation, respectively) and 17% (3/18) of nonMPN AML.
The most common mutations on next-generation sequencing included TP53, RUNX1, ASXL1, TET2, DNMT3A, SRSF2, IDH1, IDH2 and EZH2 in each group. However, of these, TP53 mutations were more frequent in MPN-BP (34% vs 11% in nonMPN AML, p<0.05) and TET2 mutations more frequent in nonMPN AML (48% vs 24% in MPN-BP, p<0.05). Interestingly, NPM1 and FLT3, which are among the most mutated genes in AML were not detected in either MPN-BP or nonMPN AML.
Based on the over-representation of erythroid/megakaryocytic phenotype in MPN-BP seen on immunohistochemistry, we hypothesized that MPN-BP blasts might exhibit an upregulated epigenetic and transcriptional program involved in erythroid/megakaryocytic differentiation. RNA-seq and ATAC-seq analysis were performed on flow cytometry sorted CD34+ leukemic blast populations from 11 MPN-BP and 6 nonMPN AML, as well as similar sorted populations from normal controls and JAK2 WT AML. Principal component analysis showed overlapping MPN-BP and nonMPN AML samples, both of which were separated from JAK2 WT AML. Differential expression analysis confirmed nearly identical gene expression between blasts from MPN-BP and nonMPN AML. In addition, consistent with findings by immunohistochemistry, the expression of genes specifically involved in erythroid/megakaryocytic differentiation (TFRC, GYPA, HBB, SPTA1, EPOR, ITGA2B, ITGB3, PF4, ALAS2, GP1BA, ENG and MPL) were upregulated in MPN-BP.
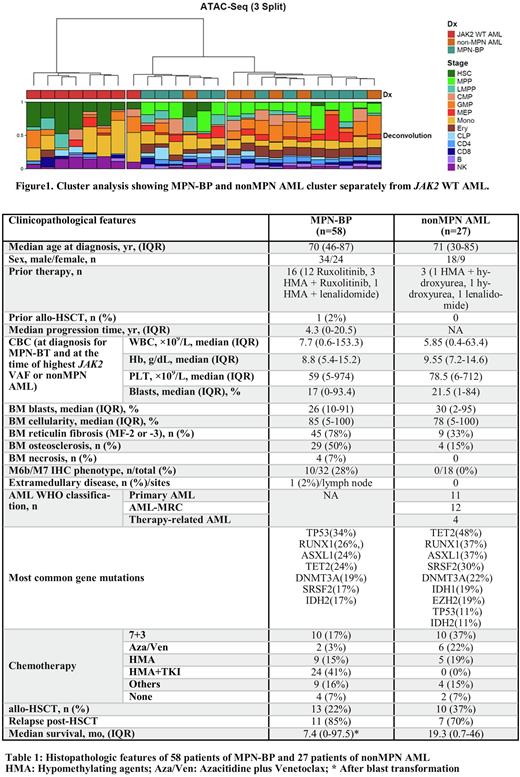
Hierarchical clustering using Ward's method on both RNA-seq and ATAC-seq normalized counts revealed that the JAK2 WT AML cluster was distinct from the MPN-BP and nonMPN AML cluster (Figure 1). Lineage deconvolution of ATAC-seq using hematopoietic differentiation stages (Glass et al., Blood 2017, Dilip et al., Blood 2021) to evaluate lineage characteristics of the blasts showed hematopoietic stem cell (HSC) predominance in JAK2 WT AML while MPN-BP and nonMPN AML displayed enrichment of multipotent progenitor (MPP), megakaryocyte-erythrocyte progenitor (MEP) and erythroid signatures.
Collectively, our data demonstrates enrichment of erythroid and megakaryocytic programs in both JAK2-mutated MPN-BP and JAK2-mutated nonMPN AML, suggesting that chromatin accessibility and transcription may be driven by mutated JAK2 regardless of antecedent MPN.
Disclosures
Galera:PAIGE.AI: Research Funding. Zhu:Leica Biosystems: Consultancy. Arcila:Bristol-Myers Squibb: Consultancy; Biocartis US, Inc: Consultancy; Clinical Care Options: Consultancy; AstraZeneca: Consultancy; Physicians’ Education Resource: Consultancy; PeerView Institute for Medical Education: Consultancy; Janssen Global Services: Consultancy; Invivoscribe: Consultancy. Dogan:Takeda: Other: Research Funding; Seattle Genetics: Consultancy; Incyte: Consultancy; Physicians’ Education Resource: Consultancy, Honoraria; Roche: Other: Research Funding; EUSA Pharma: Consultancy; Peer View: Honoraria; Loxo: Consultancy. Levine:Gilead and Novartis: Other: Grant reviews; Astra Zeneca and Kura: Other: honoraria for invited lectures ; Ajax, Abbvie, Constellation, Zenalis, Celgene, Roche, and Prelude: Other: research support; Qiagen: Other: supervisory board member; Imago, Mission Bio, Bakx, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Syndax, Incyte, Janssen, Astellas, Morphosys and Novartis: Consultancy. Roshal:Auron Therapeutics: Other: Ownership / Equity interests; Provision of services; Celgene: Other: Provision of services; Physicians’ Education Resource: Other: Provision of services; Roche: Other: Funding; NGM: Other: Funding; Beat AML: Other: Funding. Rampal:PharmaEssentia: Consultancy; Gilead: Consultancy; Stemline: Consultancy, Research Funding; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Research Funding; Zentalis: Consultancy, Research Funding; Sierra Oncology: Consultancy; Disc Medicines: Consultancy; Sunimoto Dainippon: Consultancy; Blueprint: Consultancy; Promedior: Consultancy; CTI: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy; Incyte: Consultancy, Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal